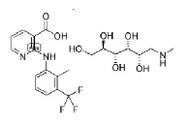
flunixin meglumine is approved for use in horses, cattle and swine; however, it is approved for use in dogs in other countries. The approved indications for its use in the horse are for the alleviation of inflammation and pain associated with musculoskeletal disorders and alleviation of visceral pain associated with colic. In cattle it is approved for the control of pyrexia associated with bovine respiratory disease and endotoxemia, and control of inflammation in endotoxemia. In swine, flunixin is approved for use to control pyrexia associated with swine respiratory disease.
Flunixin has been suggested for many other indications in various species, including: Horses: foal diarrheas, shock, colitis, respiratory disease, post-race treatment, and pre- and post ophthalmic and general surgery; Dogs: disk problems, arthritis, heat stroke, diarrhea, shock, ophthalmic inflammatory conditions, pre- and post ophthalmic and general surgery, and treatment of parvovirus infection; Cattle: acute respiratory disease, acute coliform mastitis with endotoxic shock, pain (downer cow), and calf diarrheas; Swine: agalactia/hypogalactia, lameness, and piglet diarrhea. It should be noted that the evidence supporting some of these indications is equivocal and flunixin may not be appropriate for every case.